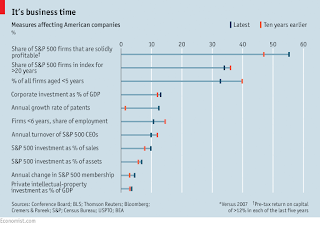
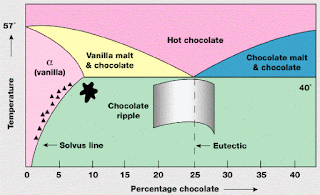
A basic but important skill: critical reading of experimental papers

Previously, I highlighted the important but basic skill of being skeptical. Here I expand on the idea. An experimental paper may make a claim, "We have observed interesting/exciting/exotic effect C in material A by measuring B." How do you critically assess such claims? Here are three issues to consider. It is as simple as ABC! 1. The material used in the experiment may not be pure A. Preparing pure samples, particularly "single" crystals of a specific material of know chemical composition is an art. Any sample will be slightly inhomogeneous and will contain some chemical impurities, defects, ... Furthermore, samples are prone to oxidation, surface reconstruction, interaction with water, ... A protein may not be in the native state... Even in a ultracold atom experiment one may have chemically pure A, but the actual density profile and temperature may not be what is thought. There are all sorts of checks one can do to characterise the structure and chemic