My most popular blogposts for 2013
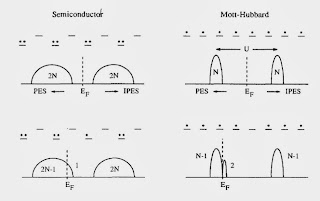
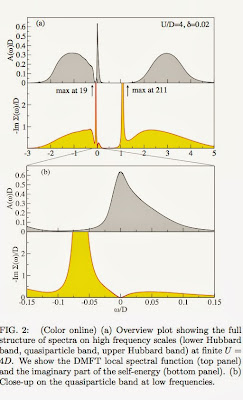
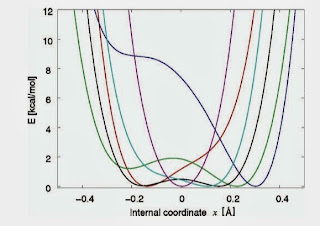
According to blogspot here are the six most popular posts from this blog over the past year Effective "Hamiltonians" for the stock market Thirty years ago in Princeton Relating non-Fermi liquid transport properties to thermodynamics Mental health talk in Canberra What simple plotting software would you recommend? A political metaphor for the correlated electron community Thanks to my readers, particularly to those who write comments. Best wishes for the New Year!